Introduction¶
Differential Thermal Analysis¶
Differential thermal analysis (DTA) is a thermal analysis technique that involves measuring the difference in temperature between a material of interest (the sample) and an inert reference material (the reference), as both are heated/cooled under identical, controlled conditions.
Materials, when heated, will undergo phase transitions at certain temperatures, that are characteristic of the given material’s underlying chemical structure. A common example of this is the melting point of ice, at 0°C, as well as the boiling point of water, at 100°C, shown in the figure below [1]:
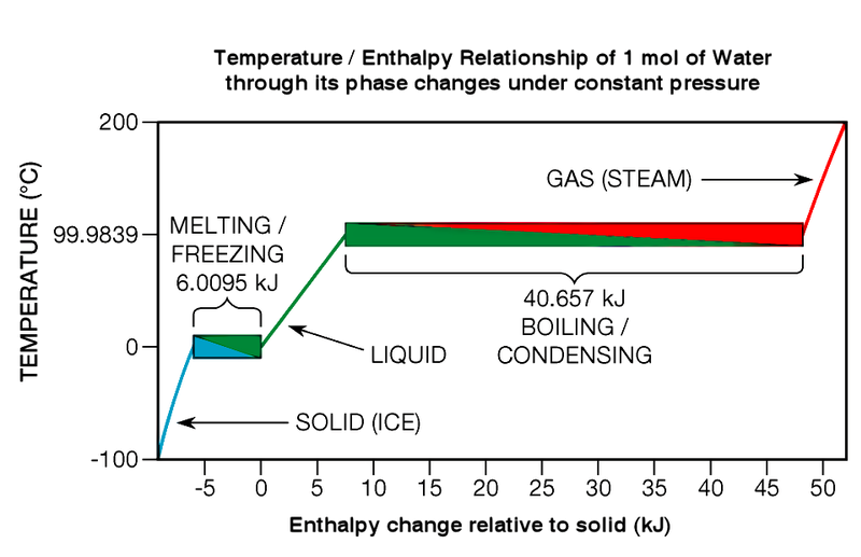
We can see that at the melting and boiling point transitions, there are large changes in the temperature-enthalpy curve - shown by the horizontal sections - due to the latent heat, which must be overcome before the temperature begins to rise again. This can be seen on a DTA curve as a temporary peak (or trough) in the temperature difference between the sample and reference.
When plotted as a function of the sample’s temperature, one can evaluate both the temperature at which a phase transition occurs, as well as the enthalpy of the transition, by accounting for the heat capacities of the sample and reference.
DTA Instrument¶
The typical construction of a DTA instrument is shown in the figure below.
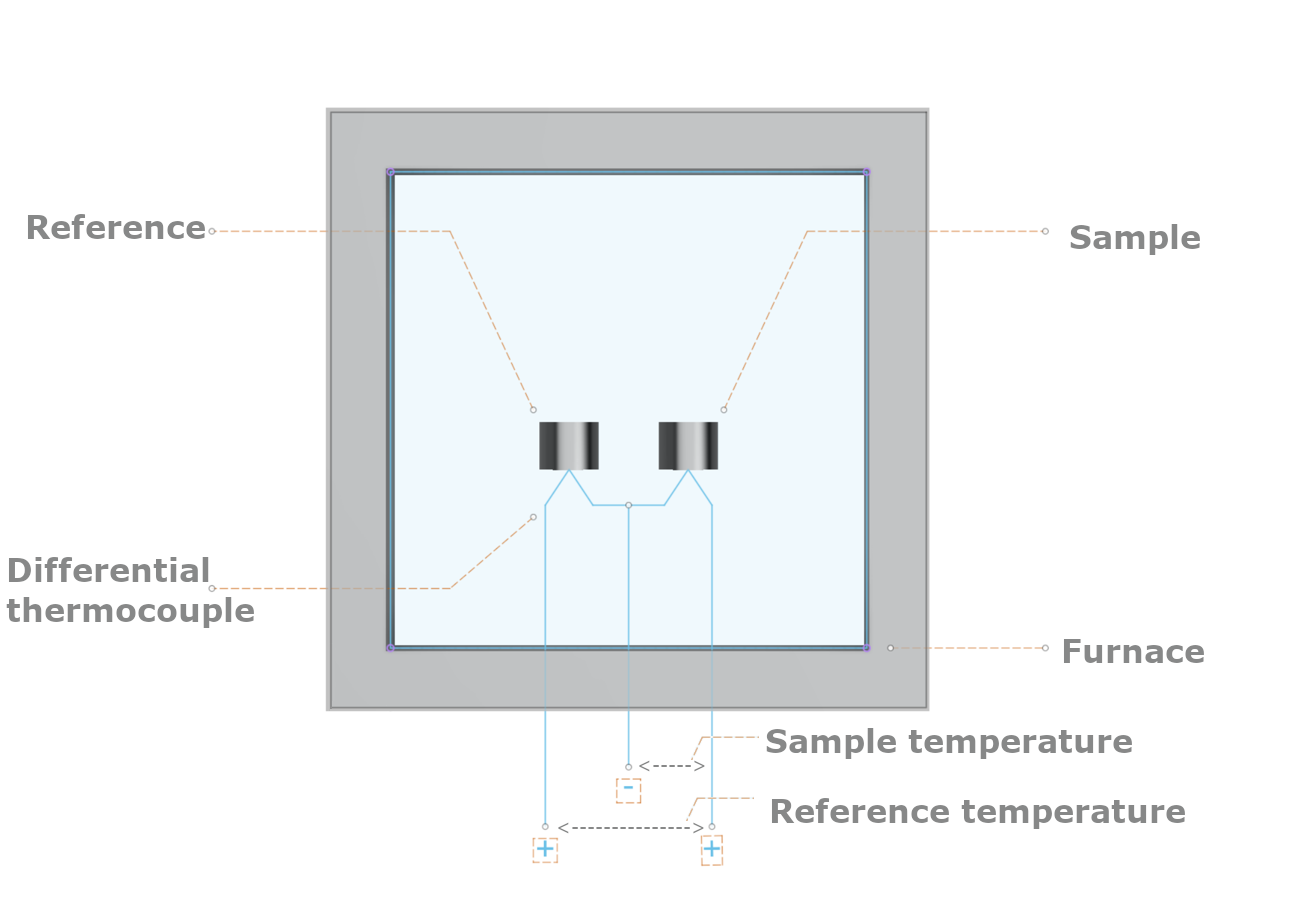
Basic features of a DTA instrument¶
The sample and reference are placed in heating pans and heated by a furnace, following a programmed temperature profile. A differential thermocouple measures the temperature difference between the sample and reference, with an additional thermocouple measuring the temperature of the reference, resulting in a temperature profile typical of that shown below:
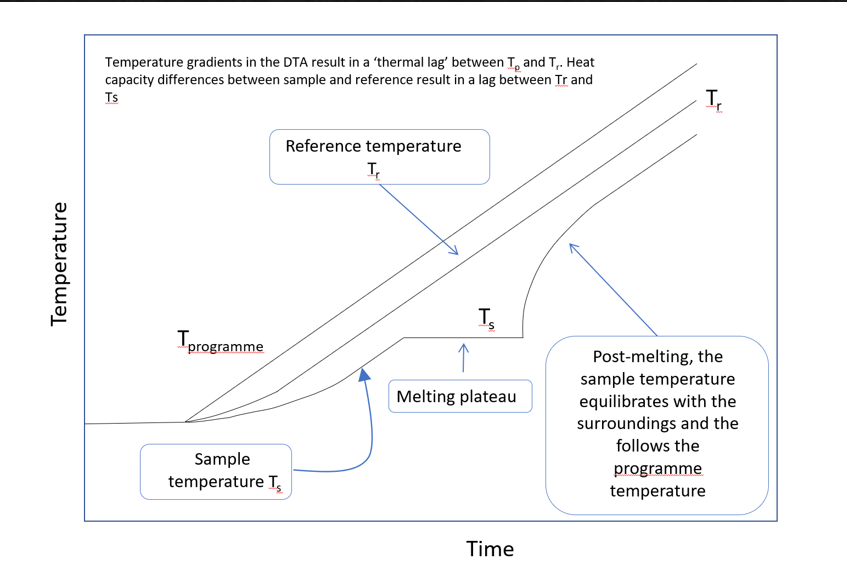
Temperature profile of sample and reference, during heating run¶
References